UPMC Digital Pathology CME Courses
CME Credit Hours: 0.50
Target Audience:
Pathologists, Pathology Residents, Medical Students, Pathology Assistants and Pathology Assistant Students
Educational Objectives:
Upon completion of this activity, participants will be able to:
- As a result of participation in this activity, participants will be able to increase awareness of the pathologic evaluation of metastatic lung adenocarcinoma
Additional Readings:
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality - All COD, Aggregated With State, Total U.S. (1969-2019), National Cancer Institute, DCCPS, Surveillance Research Program, released April 2021. Underlying mortality data provided by NCHS (www.cdc.gov/nchs).
- Cibas, Edmund S and Ducatman, Barbara S. Cytology : diagnostic principles and clinical correlates. Philadelphia, PA : Elsevier/Saunders, [2014].
- National Comprehensive Cancer Network. https://www.nccn.org/. NCCN Guidelines Version 3.2022; Non-Small Cell Lung Cancer. Date: 2022 (15 April 2022, date last accessed).
- WHO Classification of Tumours Editorial Board. Thoracic tumours [Internet]. Lyon (France): International Agency for Research on Cancer; 2021 [cited 2022 Apr 15]. (WHO classification of tumours series, 5th ed.; vol. 5). Available from: https://tumourclassification.iarc.who.int/chapters/35.
- Rosell R, et al. Erlotinib versus standard chemotherapy as first line treatment for European patients with advanced EGFR mutation positive non small cell lung cancer (EURTAC): a multicentre, open label, randomised phase 3 trial. Lancet Oncol 2012;13:239-246.
- Planchard D, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 2017;18:1307-1316.
- Skoulidis F, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med 2021;384:2371-2381.
- Wolf J, et al; GEOMETRY mono-1 Investigators. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med 2020;383:944-957.
- Drilon A, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med 2018;378:731-739.
- Soria JC, et al. Osimertinib in untreated EGFR-mutated advanced non-small cell lung cancer. N Engl J Med 2018;378:113-125.
- Cho JH, et al. Osimertinib for patients with non-small-cell lung cancer harboring uncommon EGFR mutations: a multicenter, open-label, phase II trial (KCSGLU15-09). J Clin Oncol. 2020;38:488-495
- Sequist LV, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011 Mar 23;3(75):75ra26. doi: 10.1126/scitranslmed.3002003. PMID: 21430269; PMCID: PMC3132801.
- Piotrowska Z, et al. Landscape of acquired resistance to osimertinib in EGFR-mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU-667 for acquired RET fusion. Cancer Discov. 2018;8:1529-39.
- Mehlman C, Cadranel J, Rousseau-Bussac G, Lacave R, Pujals A, Girard N, et al. Resistance mechanisms to osimertinib in EGFR-mutated advanced non-small-cell lung cancer: a multicentric retrospective French study. Lung Cancer. 2019;137:149-56.
- Marcoux N, et al. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes. J Clin Oncol. 2019 Feb 1;37(4):278-285. doi: 10.1200/JCO.18.01585. Epub 2018 Dec 14. PMID: 30550363; PMCID: PMC7001776.
- Offin M, et al. Concurrent RB1 and TP53 Alterations Define a Subset of EGFR-Mutant Lung Cancers at risk for Histologic Transformation and Inferior Clinical Outcomes. J Thorac Oncol. 2019 Oct;14(10):1784-1793. doi: 10.1016/j.jtho.2019.06.002. Epub 2019 Jun 19. PMID: 31228622; PMCID: PMC6764905.
Authors:
Jeff Kleinberger, MD, PhD and Gabriel Sica, MD, PhD
Release Date: 2022-07-08
Review Date: 2024-07-08
Expiration Date: 2025-07-08
No relationships with industry relevant to the content of this educational activity have been disclosed.
The University of Pittsburgh School of Medicine is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
The University of Pittsburgh School of Medicine designates this enduring material for a maximum of 0.50 AMA PRA Category 1 CreditsTM. Each physician
should only claim credit commensurate with the extent of their participation in the activity.
Other health care professionals are awarded ( 0.05 ) continuing education units (CEU) which are equivalent to 0.50 contact hours.
Presenters for this program have been requested to identify financial or other relationships with manufacturer(s) of any commercial product(s) or with provider(s)
of any commercial service(s) which, in the context of their topics, could be perceived as real or apparent conflicts of interest.
The University of Pittsburgh is an affirmative action, equal opportunity institution.
Case 1097 - A Man in His 60's with a History of Metastatic Lung Adenocarcinoma
Contributed by: Jeff Kleinberger, MD, PhD and Gabriel Sica, MD, PhD
A maximum of 0.50 AMA PRA Category 1 Credits are available from a quiz on this case from the University of Pittsburgh's Internet-based Studies in Education and Research.
This course is eligible for the American Board of Pathology SAM credit
Click here to take the quiz and
earn 0.50 AMA PRA Category 1 Credits
Please review the case before taking the quiz. The site will now ask for your ABPath MOC number before taking the quiz if it was not supplied previously.
CLINICAL HISTORY
A man in his 60's with a history of metastatic lung adenocarcinoma presents with progression of disease identified by positron emission tomography (PET) scan. He was initially diagnosed with lung cancer 4 years prior, when a 5.0 cm left infrahilar mass and left hilar lymphadenopathy was identified. At that time, cytologic evaluation of an endobronchial ultrasound-guided fine needle aspiration of his subcarinal lymph node demonstrated metastatic lung adenocarcinoma (Figure 1). Next-generation sequencing of the tumor using an oncologic hotspot panel identified an EGFR exon 19 deletion (p.E746_A750del), but no other mutations. Fluorescence in-situ hybridization studies (FISH) did not identify ALK rearrangement, ROS1 translocation, RET translocation, or MET amplification. Immunohistochemistry showed PD-L1 was expressed on 1% of the malignant cells. The tumor was classified as unresectable stage IIIC lung adenocarcinoma, cT3 N3.
Figure 1: High Magnification Images of Endobronchial Ultrasound Guided Fine-Needle Aspiration of Subcarinal Lymph Node, Initial Diagnosis
A: Hematoxylin and eosin stain of cell block material, B: Diff-Quik stain of cytologic specimen,
C: Synaptophysin immunohistochemical stain, D: TTF-1 immunohistochemical stain
The patient was treated with osimertinib and had a good initial response that was followed by interval increase in the size of the primary tumor and possible metastases to the bone. Osimertinib was paused and replaced with a carboplatin/etoposide regimen and radiation therapy, which resulted in a good initial response. A month later he had a cerebrovascular accident, and during work up, several brain metastases were discovered. Subsequent PET scan identified new metastases in the right adrenal gland and left iliac bone, leading to his current presentation.
Biopsy of the FDG-avid right adrenal gland mass was performed. Figure 2 shows the adrenal gland mass biopsy and immunohistochemical stain work up.
Figure 2: High Magnification Images of Ultrasound Guided Biopsy and Touch Prep of FDG-Avid Adrenal Mass, current specimen
A: Hematoxylin and eosin stain of biopsy, B: Diff-Quik stain of touch prep,
C: Synaptophysin immunohistochemical stain, D: TTF-1 immunohistochemical stain
Repeat next generation sequencing using the same oncologic hotspot panel on the adrenal mass identified the same EGFR exon 19 deletion (p.E746_A750del), but also mutations in TP53 (p.R337C) and RB1 (frameshift mutation).
What is your diagnosis?
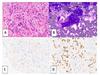 Figure 1 |
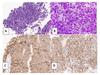 Figure 2 |
Please click the "Final Diagnosis" tab at the top of the screen to view the rest of the case.
FINAL DIAGNOSIS
Histologic transformation to small cell lung carcinoma from an EGFR-mutated lung adenocarcinoma, a mechanism of resistance to EGFR tyrosine kinase inhibitor treatment.
DISCUSSION
Lung cancer is one of the deadliest forms of cancer, accounting for 21.7% of all cancer deaths while making up only 12.4% of new cancer cases in 2021.[1] Non-small cell carcinoma (adenocarcinoma, squamous cell carcinoma, and large-cell carcinoma) and small cell carcinoma account for the majority of types of lung cancer. Small cell lung carcinoma (SCLC) is often treated initially with systemic chemotherapy due to its aggressiveness and advanced stage at detection. It is strongly associated with smoking history with less than 10% 5-year survival rate, largely due to advanced stage at presentation and lack of significant advances in therapeutics; although this may be changing with the emergence of immunotherapy.[2] In contrast, because there are multiple FDA approved agents available for patients with advanced stage adenocarcinoma based on the oncogenic profile of a lung adenocarcinoma, molecular profiling of lung adenocarcinoma, especially the more advanced staged adenocarcinomas, are standard of care. Guidelines now suggest that extensive immunohistochemical workup should be avoided in small biopsies to save material for molecular studies.[3] This case demonstrates the interesting relationship between histological findings, molecular data, and therapy regimens.
Lung adenocarcinomas (LUAD) in cytologic preparations have an appearance similar to many other types of adenocarcinomas. The cells form three-dimensional clusters with eccentrically placed, irregular nuclei, prominent nucleoli, and mucin vacuoles.[2] On biopsy specimens, the cells of non-mucinous adenocarcinoma can display lepidic, acinar, papillary, micropapillary, cribriform, and solid architectural patterns, often with a combination of patterns present. Evidence of invasion includes non-lepidic patterns, invasive tumor cells within stroma, vascular invasion, pleural invasion, or spread through airspace. Tumors measuring greater than 2.5cm, containing solid architectural pattern, or containing micropapillary architectural pattern generally have a worse prognosis.[4] Evaluation using a minimal number of immunohistochemical stains, such as only p40 and TTF-1, is suggested to demonstrate that the tumor cells are of lung origin/adenocarcinoma (e.g. TTF-1 positive) and are non-squamous (e.g. p40 negative), while preserving material for molecular studies.[3]
Molecular profiling is essential for advanced stage LUAD treatment decision planning since the choice of chemotherapy, immunotherapy, or targeted therapy as a first line systemic treatment can be based on the presence of driver oncogene molecular alterations that have FDA approved inhibitors; such oncogenes in LUAD include EGFR, ALK, ROS1, BRAF, KRAS, MET, and NTRK1/2/3. Activating mutations in EGFR are most commonly p.L858R or exon 19 deletions, and they are seen in up to 15% of LUAD (more frequent in never-smokers).[5] Gene rearrangements of ALK, ROS1, or RET, which can all be detected by next generation sequencing or FISH studies, are also responsive to select tyrosine kinase inhibitors (TKIs), based on the affected gene. A specific mutation in BRAF, p.V600E, is responsive to combinations of BRAF and MEK inhibitors (such as dabrafenib and trametinib).[6] Mutations in KRAS generally suggest a poor prognosis and less responsiveness to TKI therapy, although a specific p.G12C mutation may be treated with sotorasib.[7] Mutations that cause exon 14 skipping in the MET gene are responsive to a MET TKI, like capmatinib.[8] Finally, gene fusions containing NTRK1/2/3 are rare in LUAD, but are associated with responsiveness to TRK inhibitors (larotrectinib, entrectinib).[9]
The patient in this case had a LUAD with a common type of EGFR mutation, an exon 19 deletion, which was treated with osimertinib, a third generation EGFR TKI. Osimertinib treatment is associated with improved survival benefits in the setting of EGFR-mutated LUAD in both EGFR treatment naïve patients and patients previously treated with EGFR TKI who have developed p.T790M resistance mutations.[10, 11] Treatment resistance to first generation EGFR TKIs are most commonly associated with additional mutations in EGFR (e.g. p.T790M for first generation TKI, p.C797S for osimertinib), bypass signal activation such as gene copy amplification of the MET gene, and histologic transformation to small cell carcinoma.[12] For osimertinib, histologic transformation to squamous cell carcinoma occurs as well.[13,14]
Up to 10% of EGFR-mutant LUAD undergo transformation to SCLC for first generation EGFR TKIs and is also an important mechanism of resistance to osimertinib. In patients who had histologic transformation to SCLC in the setting of first generative EGFR TKI therapy, the median time to transformation in one study was 17.8 months.[15] That study showed that transformed SCLC has behavior clinically similar to those de novo SCLC (non-EGFR mutated), with transient therapy response, frequent CNS metastasis, and short overall survival time (Figure 3). [15]
SCLC often has TP53 and RB1 genomic alterations, among many other types of driver mutations. While this case demonstrated a tumor that acquired those mutations at the same time as the histologic transformation, only approximately 25% of LUAD that have EGFR, RB1, and TP53 mutations underwent transformation in one retrospective study (Figure 4).[16]
Figure 3: Survival curves of time to transformation and overall survival in SCLC transformed from EGFR-mutated LUAD.
From: Marcoux N, et al. J Clin Oncol. 2019.[15] Survival curves demonstrating the time to transformation (A) and overall survival post-transformation (D) in a retrospective study of 67 cases of EGFR-mutated LUAD that underwent transformation into SCLC.
Small cell carcinoma has a unique cytologic presentation in which cells are small with minimal cytoplasm, molding and nuclei with "powdery" chromatin without distinct nucleoli. Mitoses should be readily apparent. There is often cellular debris, crush artifact, and some paranuclear blue bodies. The cytologic preparation in Figure 2 of this case demonstrates powdery chromatin, some nuclear molding, and crush artifact. SCLC must be differentiated from carcinoid tumor, atypical carcinoid tumor, lymphoid aggregations, reserve cell hyperplasia, and small round blue cell tumors.[2] Immunohistochemical stains on SCLC show a rim-and-dot-type pattern for AE1/AE3 and CAM5.2, positivity for neuroendocrine markers (synaptophysin, chromogranin, and CD56), positivity for TTF-1, negativity for Napsin A, and negativity for p63 or p40. Ki-67 proliferative index is usually very high (65-100%). Consistent with the molecular findings in SCLC, IHC usually shows loss of RB1 and aberrant p53 expression.[4]
Figure 4: Histologic patterns and additional mutations in lung cancers containing EGFR, RB1, and TP53 mutations.
From: Offin M, et al. J Thorac Oncol. 2019. [16]
This case demonstrates an EGFR-mutated LUAD transformation to SCLC, likely in response to TKI therapy. It highlights the importance of correlations between histologic findings and prognosis, molecular findings and treatment, and treatment-related resistance mechanisms both histologically and through molecular mechanisms.
 Figure 3 |
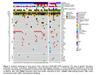 Figure 4 |
Click here to take the quiz and
earn 0.50 AMA PRA Category 1 Credits
Please review the case before taking the quiz. The site will now ask for your ABPath MOC number before taking the quiz if it was not supplied previously.