UPMC Digital Pathology CME Courses
CME Credit Hours: 0.50
Target Audience:
Pathologists, Pathology Residents, Medical Students, Pathology Assistants and Pathology Assistant Students
Educational Objectives:
Upon completion of this activity, participants will be able to:
- As a result of participation in this activity, participants will be able to increase their awareness of rare neuropathologic entities.
Additional Readings:
- Di Russo P, Perrini P, Pasqualetti F, Meola A, Vannozzi R. Management and outcome of high-grade multicentric gliomas: a contemporary single-institution series and review of the literature. Acta Neurochir (Wien). 2013 Dec;155(12):2245-51.
- Terakawa Y, Yordanova YN, Tate MC, Duffau H. Surgical management of multicentric diffuse low-grade gliomas: functional and oncological outcomes: clinical article. J Neurosurg. 2013 Jun;118(6):1169-75.
- Vaubel RA, Kollmeyer TM, Caron AA, et al. Synchronous gemistocytic astrocytoma IDH-mutant and oligodendroglioma IDH-mutant and 1p/19q-codeleted in a patient with CCDC26 polymorphism. Acta Neuropathol. 2017 Aug;134(2):317-319.
- Oktay Y, Ülgen E, Can Ö, et al. IDH-mutant glioma specific association of rs55705857 located at 8q24.21 involves MYC deregulation. Sci Rep. 2016 Jun 10;6:27569.
- González-Castro TB, Juárez-Rojop IE, López-Narváez ML, et al. Genetic Polymorphisms of CCDC26 rs891835, rs6470745, and rs55705857 in Glioma Risk: A Systematic Review and Meta-analysis. Biochem Genet. 2019 Aug;57(4):583-605.
- Huse JT, Diamond EL, Wang L, Rosenblum MK. Mixed glioma with molecular features of composite oligodendroglioma and astrocytoma: a true "oligoastrocytoma"? Acta Neuropathol. 2015 Jan;129(1):151-3.
- Wilcox P, Li CC, Lee M, Shivalingam B, et al. Oligoastrocytomas: throwing the baby out with the bathwater? Acta Neuropathol. 2015 Jan;129(1):147-9
Authors:
Contributed by Wen Zhong, MD and Tom Pearce, MD, PhD
Release Date: 2022-04-16
Review Date: 2022-04-18
Expiration Date: 2025-04-16
No relationships with industry relevant to the content of this educational activity have been disclosed.
The University of Pittsburgh School of Medicine is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
The University of Pittsburgh School of Medicine designates this enduring material for a maximum of 0.50 AMA PRA Category 1 CreditsTM. Each physician
should only claim credit commensurate with the extent of their participation in the activity.
Other health care professionals are awarded ( 0.05 ) continuing education units (CEU) which are equivalent to 0.50 contact hours.
Presenters for this program have been requested to identify financial or other relationships with manufacturer(s) of any commercial product(s) or with provider(s)
of any commercial service(s) which, in the context of their topics, could be perceived as real or apparent conflicts of interest.
The University of Pittsburgh is an affirmative action, equal opportunity institution.
Case 1086 - A Man in His 30's with Brain Lesions
Contributed by: Contributed by Wen Zhong, MD and Tom Pearce, MD, PhD
A maximum of 0.50 AMA PRA Category 1 Credits are available from a quiz on this case from the University of Pittsburgh's Internet-based Studies in Education and Research.
This course is eligible for the American Board of Pathology SAM credit
Click here to take the quiz and
earn 0.50 AMA PRA Category 1 Credits
Please review the case before taking the quiz. The site will now ask for your ABPath MOC number before taking the quiz if it was not supplied previously.
CLINICAL HISTORY
A man in his 30's presented to the emergency department following a tonic-clonic seizure with generalization. He had no history of prior seizures and denied any trauma, vision changes, imbalance, memory issues, weakness or paresthesia. Brain MRI discovered two separate areas of expansile T2 FLAIR hyperintensity, with discrete lesions seen in the left frontal lobe and right medial parietooccipital region. Neither lesion showed enhancement following administration of contrast. A CT scan of the chest, abdomen and pelvis revealed no evidence of primary malignancy. Given the radiological characteristics, multifocal glioma was suspected, with a differential diagnosis of acute disseminated encephalomyelitis and nonenhancing tumefactive multiple sclerosis. The patient subsequently underwent craniotomies for resection of both lesions with a 6-week interval.
MICROSCOPIC DESCRIPTION
H&E stained sections of the left frontal lesion revealed a diffusely infiltrating glial neoplasm involving cortex and white matter. Many tumor cells had bland cytomorphology, but in some areas, increased nuclear pleomorphism and frequent gemistocytic appearance were appreciated. Mitotic figures were identified but necrosis and endothelial proliferation were absent. Sections from the right parietooccipital lesion also consisted with an infiltrating glial neoplasm composed of cells exhibiting round to ovoid nuclei with mild nuclear atypia. There were very sparse mitotic figures with no evidence of endothelial proliferation or tumor necrosis.
Immunohistochemically, many tumor cells in the frontal lesion showed starburst-like staining pattern by GFAP, which was different from the parietooccipital lesion, where a diffuse GFAP positivity was observed. Both neoplasms demonstrated diffuse expression of IDH1 R132H, but a positive clonal staining pattern of p53 protein was only seen in the frontal lesion, with nuclear positivity in greater than 70% of tumor cells, compared to faint nuclear staining in a subset of tumor cells in the parietooccipital lesion. ATRX in the frontal lesion demonstrated a biphasic pattern with decreased but not complete loss of nuclear staining in tumor cells with preserved strong nuclear reactivity in background non-neoplastic cells, whereas the nuclear positivity of ATRX was strongly retained in both tumor and normal in the parietooccipital lesion. The Ki67 proliferation index was considerably higher in the frontal lesion (up to 10%) than in the parietooccipital lesion (1% overall).
Molecular analysis was performed on both neoplasms. In addition to IDH1 R132H mutation predicted by immunohistochemical staining, the frontal lesion additionally harbored a TP53 mutation. Despite the abnormal ATRX immunostaining pattern, no ATRX mutation was identified. In contrast to the frontal lesion, the parietooccipital lesion contained a TERT promoter mutation, 1p/19q copy number loss and MGMT promoter methylation. Aside from IDH1 R132H, the two tumors did not share any additional pathologic mutations, insertions/deletions, or copy number alterations.
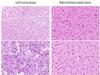 HE |
 IHC |
 Table |
PATHOLOGICAL FINDINGS
Axial MRI T2 FLAIR: an expansile mass-like T2/FLAIR hyperintensity is seen in the centrum semiovale underneath the left superior frontal middle gyri (4x3 cm) and a second lesion is located in the medial aspect of the right occipital lobe at the region of the splenium (4x2 cm). There was abnormal parenchymal diffusion restriction, midline shift, or post-contrast enhancement (not shown). The intervening tissue between the lesions showed no MRI signal abnormalities.
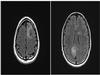 Radiology Image |
Please click the "Final Diagnosis" tab at the top of the screen to view the rest of the case.
FINAL DIAGNOSIS
Synchronous IDH-mutant anaplastic astrocytoma and oligodendroglioma:
Left Frontal Lesion: ANAPLASTIC ASTROCYTOMA, IDH-mutant, WHO grade 3;
Right Parietooccipital Lesion: OLIGODENDROGLIOMA, IDH-mutant and 1p/19q co-deleted, WHO grade 2
DISCUSSION
IDH-mutant gliomas occur most often in adults between the ages of 30-50 and present as single parenchymal lesion. Multicentric gliomas are defined as multiple anatomically separated lesions, located in different lobes or hemispheres, with no apparent hematogenous or CSF dissemination or local spread of tumor cells. This clinical and radiologic picture is typically encountered in high-grade tumors such as glioblastomas and is a well-documented phenomenon, reported in 2-16.2% of cases [1]. Despite the lack of MRI signal abnormalities in the intervening brain tissue, at the histopathologic level these tumors can be widely invasive, with sparse malignant cells invading far from the main tumor mass. In many cases, therefore, multicentric gliomas likely arise from a single tumor cell population that expands into a radiologically identifiable mass in multiple locations.
In contrast, the simultaneous development of two primary gliomas with different histological types and distinct molecular profiles is much rarer and the etiology is unclear. In a small case series of multicentric diffuse low-grade gliomas, a 35-year-old man with WHO Grade II oligodendroglioma and astrocytoma found in separate hemispheres was described [2]. Most recently, Vaubel and colleagues reported a synchronous gemistocytic astrocytoma and oligodendroglioma in a 49-year-old man harboring rs55705857 polymorphism at 8q24 near CCDC26 [3], a risk allele associated with IDH-mutant gliomas [4][5]. Several theories have been proposed attempting to explain the synchronicity of primary brain tumors with different phenotypes and genotypes, but they are largely speculative. Some authors have postulated that an oncogenic stimulus could yield a malignant transformation in different cell lineages or could cause a transformation only in susceptible regions. Others have suggested that one neoplasm may act as an irritant which stimulates the development of the other. Another possibility that should be taken into consideration, especially in biopsy only cases, is intratumoral heterogeneity, which has been discussed in rare dual-genotype oligoastrocytomas with shared IDH alteration and biphasic components harboring ATRX/TP53 mutation and 1p/19q-codeletion, respectively [6][7].
In our case, the lesions demonstrated distant locations, different phenotypes and WHO grades, and distinct molecular profiles with IDH1 mutation being the only shared feature. Although the presence of a common progenitor population with only IDH1 mutation cannot be entirely excluded, we do not have clear evidence of such a population, and we favor the random synchronous development of two independent IDH-mutant glial neoplasms. Whether this could be influenced in the current case by a risk allele predisposing to IDH-mutant tumors has not been tested.
REFERENCES
Di Russo P, Perrini P, Pasqualetti F, Meola A, Vannozzi R. Management and outcome of high-grade multicentric gliomas: a contemporary single-institution series and review of the literature. Acta Neurochir (Wien). 2013 Dec;155(12):2245-51.
Terakawa Y, Yordanova YN, Tate MC, Duffau H. Surgical management of multicentric diffuse low-grade gliomas: functional and oncological outcomes: clinical article. J Neurosurg. 2013 Jun;118(6):1169-75.
Vaubel RA, Kollmeyer TM, Caron AA, Barr Fritcher EG, Voss JS, Liang H, Jenkins RB, Giannini C, Kipp BR. Synchronous gemistocytic astrocytoma IDH-mutant and oligodendroglioma IDH-mutant and 1p/19q-codeleted in a patient with CCDC26 polymorphism. Acta Neuropathol. 2017 Aug;134(2):317-319.
Oktay Y, Ülgen E, Can Ö, Akyerli CB, Yüksel ?, Erdemgil Y, Duras? IM, Henegariu OI, Nanni EP, Selevsek N, Grossmann J, Erson-Omay EZ, Bai H, Gupta M, Lee W, Turcan ?, Özp?nar A, Huse JT, Sav MA, Flanagan A, Günel M, Sezerman OU, Yak?c?er MC, Pamir MN, Özduman K. IDH-mutant glioma specific association of rs55705857 located at 8q24.21 involves MYC deregulation. Sci Rep. 2016 Jun 10;6:27569.
González-Castro TB, Juárez-Rojop IE, López-Narváez ML, Tovilla-Zárate CA, Genis-Mendoza AD, Pérez-Hernández N, Martínez-Magaña JJ, Rodríguez-Pérez JM. Genetic Polymorphisms of CCDC26 rs891835, rs6470745, and rs55705857 in Glioma Risk: A Systematic Review and Meta-analysis. Biochem Genet. 2019 Aug;57(4):583-605.
Huse JT, Diamond EL, Wang L, Rosenblum MK. Mixed glioma with molecular features of composite oligodendroglioma and astrocytoma: a true "oligoastrocytoma"? Acta Neuropathol. 2015 Jan;129(1):151-3.
Wilcox P, Li CC, Lee M, Shivalingam B, Brennan J, Suter CM, Kaufman K, Lum T, Buckland ME. Oligoastrocytomas: throwing the baby out with the bathwater? Acta Neuropathol. 2015 Jan;129(1):147-9.
Click here to take the quiz and
earn 0.50 AMA PRA Category 1 Credits
Please review the case before taking the quiz. The site will now ask for your ABPath MOC number before taking the quiz if it was not supplied previously.