UPMC Digital Pathology CME Courses
CME Credit Hours: 0.50
Target Audience:
Pathologists, Pathology Residents, Medical Students, Pathology Assistants and Pathology Assistant Students
Educational Objectives:
Upon completion of this activity, participants will be able to:
-
As a result of participation in this activity, participants will be able to describe the pathologic and molecular features of papillary renal cell carcinoma with reverse polarity.
Additional Readings:
- Delahunt B, Eble JN, McCredie MR, Bethwaite PB, Stewart JH, Bilous AM. Morphologic typing of papillary renal cell carcinoma: comparison of growth kinetics and patient survival in 66 cases. Hum Pathol. 2001;32(6):590-595.
- Saleeb RM, Plant P, Tawedrous E, et al. Integrated Phenotypic/Genotypic Analysis of Papillary Renal Cell Carcinoma Subtypes: Identification of Prognostic Markers, Cancer-related Pathways, and Implications for Therapy. Eur Urol Focus. 2018;4(5):740-748.
- Saleeb RM, Brimo F, Farag M, et al. Toward Biological Subtyping of Papillary Renal Cell Carcinoma With Clinical Implications Through Histologic, Immunohistochemical, and Molecular Analysis. Am J Surg Pathol. 2017;41(12):1618-1629.
- Chevarie-Davis M, Riazalhosseini Y, Arseneault M, et al. The morphologic and immunohistochemical spectrum of papillary renal cell carcinoma: study including 132 cases with pure type 1 and type 2 morphology as well as tumors with overlapping features. Am J Surg Pathol. 2014;38(7):887-894.
- Trpkov K, Hes O, Williamson SR, et al. New developments in existing WHO entities and evolving molecular concepts: The Genitourinary Pathology Society (GUPS) update on renal neoplasia. Mod Pathol Off J U S Can Acad Pathol Inc. 2021;34(7):1392-1424.
- Kunju LP, Wojno K, Wolf JS, Cheng L, Shah RB. Papillary renal cell carcinoma with oncocytic cells and nonoverlapping low grade nuclei: expanding the morphologic spectrum with emphasis on clinicopathologic, immunohistochemical and molecular features. Hum Pathol. 2008;39(1):96-101.
- Al-Obaidy KI, Eble JN, Cheng L, et al. Papillary Renal Neoplasm With Reverse Polarity: A Morphologic, Immunohistochemical, and Molecular Study. Am J Surg Pathol. 2019;43(8):1099-1111.
- Wei S, Kutikov A, Patchefsky AS, et al. Papillary Renal Neoplasm With Reverse Polarity Is Often Cystic: Report of 7 Cases and Review of 93 Cases in the Literature. Am J Surg Pathol. Published online August 5, 2021. doi:10.1097/PAS.0000000000001773
- Kiyozawa D, Kohashi K, Takamatsu D, et al. Morphological, immunohistochemical, and genomic analyses of papillary renal neoplasm with reverse polarity. Hum Pathol. 2021;112:48-58. doi:10.1016/j.humpath.2021.03.009
Authors:
Contributed by Daniel Geisler, MD and Gabriela Quiroga-Garza, MD
Release Date: 2022-04-16
Review Date: 2022-04-18
Expiration Date: 2025-04-16
No relationships with industry relevant to the content of this educational activity have been disclosed.
The University of Pittsburgh School of Medicine is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
The University of Pittsburgh School of Medicine designates this enduring material for a maximum of 0.50 AMA PRA Category 1 CreditsTM. Each physician
should only claim credit commensurate with the extent of their participation in the activity.
Other health care professionals are awarded ( 0.05 ) continuing education units (CEU) which are equivalent to 0.50 contact hours.
Presenters for this program have been requested to identify financial or other relationships with manufacturer(s) of any commercial product(s) or with provider(s)
of any commercial service(s) which, in the context of their topics, could be perceived as real or apparent conflicts of interest.
The University of Pittsburgh is an affirmative action, equal opportunity institution.
Case 1084 - A Female in Her 60s with a Kidney Mass
Contributed by: Contributed by Daniel Geisler, MD and Gabriela Quiroga-Garza, MD
A maximum of 0.50 AMA PRA Category 1 Credits are available from a quiz on this case from the University of Pittsburgh's Internet-based Studies in Education and Research.
This course is eligible for the American Board of Pathology SAM credit
Click here to take the quiz and
earn 0.50 AMA PRA Category 1 Credits
Please review the case before taking the quiz. The site will now ask for your ABPath MOC number before taking the quiz if it was not supplied previously.
CLINICAL HISTORY
The patient is a female in her 60s who presents with an incidentally detected kidney mass. Computed tomography shows an enhancing 2.0 cm exophytic, solid right kidney mass of the lower pole. She denies any symptoms, smoking history, or family history of urological malignancies. The patient elects to undergo a laparoscopic partial nephrectomy of the mass in lieu of continued surveillance.
GROSS DESCRIPTION
The specimen measures 3.0 x 2.0 x 1.2 cm and consists of a well-defined, partially cauterized, enucleated, and encapsulated lesion. A scant amount of apparent normal renal parenchyma is attached. The cut surface shows a well-defined, glistening, tan-red-brown, and rubbery to spongy mass with no obvious necrosis (Figure 1). The lesion abuts both the parenchymal margin and capsular surface, without discrete involvement of either.
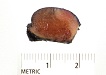 Figure 1 |
MICROSCOPIC DESCRIPTION
The lesion is well-circumscribed with varying papillary and tubulopapillary patterned architecture (Figure 2A-B). The papillae are lined by a single layer of small to medium-sized cells with eosinophilic cytoplasm. The nuclei are generally uniform and ovoid with inconspicuous nucleoli arranged away from the basal aspect of the cell (Figure 2C-D). The scant stroma is composed of hyalinized to loose fibroconnective tissue with focal edematous areas. Tumor necrosis, mitotic figures, lymphovascular invasion, and perirenal invasion are not identified. mmunohistochemical stains performed on the tumor show CK7 (Figure 3A), GATA3 (Figure 3B), and EMA (Figure 3C) positivity. The tumor cells are negative for vimentin (Figure 3D), renal cell carcinoma antigen, CD10, carbonic anhydrase IX, and CD117. Parvalbumin demonstrates patchy, weak positivity. Ki-67 shows a low proliferation index with less than 1% of tumor cells positive.
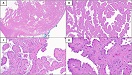 Figure 2 |
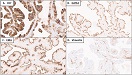 Figure 3 |
Please click the "Final Diagnosis" tab at the top of the screen to view the rest of the case.
FINAL DIAGNOSIS
Papillary renal neoplasm with reverse polarity (PRNRP)
DISCUSSION
Papillary renal cell carcinoma, the second most common renal cell carcinoma, encompasses a spectrum of tumors with different clinical, morphologic, histologic, and molecular features. Due to the poor interobserver reproducibility and overlapping histologic features, the prior division into type 1 and 2 subtypes is no longer recommended [1-5]. New tumor variants have been recently emerging within the papillary renal cell carcinoma spectrum with specific histomorphology, immunoprofile, and clinical behavior [5]. PRNRP is one such newly proposed entity that was originally recognized in 2008 and described as a favorable papillary renal cell carcinoma characterized by oncocytic cells with nonoverlapping, peripheralized low-grade nuclei [6]. Al-Obaidy et al [7] further characterized these tumors, showing consistent KRAS mutations, and proposed the name PRNRP.
Typical clinicopathologic features include a mean age of presentation of 63 years old (range, 36-82 years old), a slight male predominance (56:44 M:F ratio), and an average tumor size of 2.1 cm (range, 0.8 to 8.5 cm) [8]. PRNRP accounts for 4% of papillary renal cell carcinomas [7]. Characteristic histomorphologic findings include a well-circumscribed, solid to partially cystic tumor with predominant thin papillary to tubulopapillary growth patterns. Papillae cores are variably hyalinized to cystically dilated with floating macrophages. The epithelial lining is formed by cuboidal cells with finely granular, eosinophilic cytoplasm. The nuclei are characteristically apically located, away from the basement membrane, and show lower Fuhrman and WHO/International Society of Urological Pathology (ISUP) nuclear grades of 1-2. Necrosis, mitotic figures, psammoma bodies, and tight clusters of foamy macrophages are absent [6-11]. The immunohistochemical profile of PRNRP shows CKAE1/AE3, CK7, GATA3, EMA, and L1CAM positivity. CD117 and vimentin are consistently negative. Alpha-mehtylacyl-CoA-racemase (AMACR) and CD10 shows variable staining [5-12]. Unique KRAS missense mutations have been identified in 85% of cases tested, with the most common KRAS mutation being p.G12V in 54% of cases [7, 8, 11, 12]. FISH analysis has shown trisomy 7, trisomy 17, and chromosome Y deletion in 33%, 33%, and 14% of PRNRP cases, which is more classically associated with other papillary renal cell carcinomas [7]. Most cases present with low pathologic stage (pT1a, 97%), and patient follow-up has shown no recurrence, metastasis, or tumor-related death during the follow up period [7, 8, 11].
The differential diagnosis includes kidney neoplasms with eosinophilic cytoplasm and papillary growth patterns. High grade eosinophilic kidney neoplasms with papillary growth could include fumarate hydratase-deficient renal cell carcinoma, MiT/TFE family translocation renal cell carcinomas, acquired cystic disease-associated renal cell carcinoma, and other renal papillary carcinoma variants. These tumors, however, will show high grade histologic features, including high WHO/ISUP nuclear grade. Tumors in the low grade spectrum that can mimic PRNRP include oncocytoma and chromophobe carcinoma, however, these tumors will not typically show a papillary growth pattern and will be positive for CD117. Other low-grade papillary renal cell carcinomas or morphologic variants of clear cell renal cell carcinoma may also be included in the differential, and ancillary immunohistochemical or molecular testing to show classic PRNRP CK7 and GATA3 positivity or KRAS mutations may be helpful in these scenarios.
REFERENCES
Delahunt B, Eble JN, McCredie MR, Bethwaite PB, Stewart JH, Bilous AM. Morphologic typing of papillary renal cell carcinoma: comparison of growth kinetics and patient survival in 66 cases. Hum Pathol. 2001;32(6):590-595. doi:10.1053/hupa.2001.24984
Saleeb RM, Plant P, Tawedrous E, et al. Integrated Phenotypic/Genotypic Analysis of Papillary Renal Cell Carcinoma Subtypes: Identification of Prognostic Markers, Cancer-related Pathways, and Implications for Therapy. Eur Urol Focus. 2018;4(5):740-748. doi:10.1016/j.euf.2016.09.002
Saleeb RM, Brimo F, Farag M, et al. Toward Biological Subtyping of Papillary Renal Cell Carcinoma With Clinical Implications Through Histologic, Immunohistochemical, and Molecular Analysis. Am J Surg Pathol. 2017;41(12):1618-1629. doi:10.1097/PAS.0000000000000962
Chevarie-Davis M, Riazalhosseini Y, Arseneault M, et al. The morphologic and immunohistochemical spectrum of papillary renal cell carcinoma: study including 132 cases with pure type 1 and type 2 morphology as well as tumors with overlapping features. Am J Surg Pathol. 2014;38(7):887-894. doi:10.1097/PAS.0000000000000247
Trpkov K, Hes O, Williamson SR, et al. New developments in existing WHO entities and evolving molecular concepts: The Genitourinary Pathology Society (GUPS) update on renal neoplasia. Mod Pathol Off J U S Can Acad Pathol Inc. 2021;34(7):1392-1424. doi:10.1038/s41379-021-00779-w
Kunju LP, Wojno K, Wolf JS, Cheng L, Shah RB. Papillary renal cell carcinoma with oncocytic cells and nonoverlapping low grade nuclei: expanding the morphologic spectrum with emphasis on clinicopathologic, immunohistochemical and molecular features. Hum Pathol. 2008;39(1):96-101. doi:10.1016/j.humpath.2007.05.016
Al-Obaidy KI, Eble JN, Cheng L, et al. Papillary Renal Neoplasm With Reverse Polarity: A Morphologic, Immunohistochemical, and Molecular Study. Am J Surg Pathol. 2019;43(8):1099-1111. doi:10.1097/PAS.0000000000001288
Wei S, Kutikov A, Patchefsky AS, et al. Papillary Renal Neoplasm With Reverse Polarity Is Often Cystic: Report of 7 Cases and Review of 93 Cases in the Literature. Am J Surg Pathol. Published online August 5, 2021. doi:10.1097/PAS.0000000000001773
Kiyozawa D, Kohashi K, Takamatsu D, et al. Morphological, immunohistochemical, and genomic analyses of papillary renal neoplasm with reverse polarity. Hum Pathol. 2021;112:48-58. doi:10.1016/j.humpath.2021.03.009
Zhou L, Xu J, Wang S, et al. Papillary Renal Neoplasm With Reverse Polarity: A Clinicopathologic Study of 7 Cases. Int J Surg Pathol. 2020;28(7):728-734. doi:10.1177/1066896920918289
Kim SS, Cho YM, Kim GH, et al. Recurrent KRAS mutations identified in papillary renal neoplasm with reverse polarity-a comparative study with papillary renal cell carcinoma. Mod Pathol. 2020;33(4):690-699. doi:10.1038/s41379-019-0420-8
Chang HY, Hang JF, Wu CY, et al. Clinicopathological and molecular characterisation of papillary renal neoplasm with reverse polarity and its renal papillary adenoma analogue. Histopathology. 2021;78(7):1019-1031. doi:10.1111/his.14320
Click here to take the quiz and
earn 0.50 AMA PRA Category 1 Credits
Please review the case before taking the quiz. The site will now ask for your ABPath MOC number before taking the quiz if it was not supplied previously.